Regardless of the crop, the basis for all fertility programs should be the 4 Rs: The Right nutrient applied in the Right place at the Right rate at the Right time. The collection of soil samples and their analysis provides important information to help achieve these four fundamentals.1
Macronutrients required by actively growing soybean plants include nitrogen (N), phosphorus (P), and potassium (K). Required secondary and micronutrients include calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), boron (B), manganese (Mn), zinc (Zn), copper (Cu), and molybdenum (Mo). Additionally, soil pH levels for soybean should be maintained between 5.5 and 7.0 to help maintain overall soil nutrient availability; however, the optimal range is between 6.3 and 6.5. The availability of different nutrients during the growing season is greatly dependent on soil pH (Figure 1).2
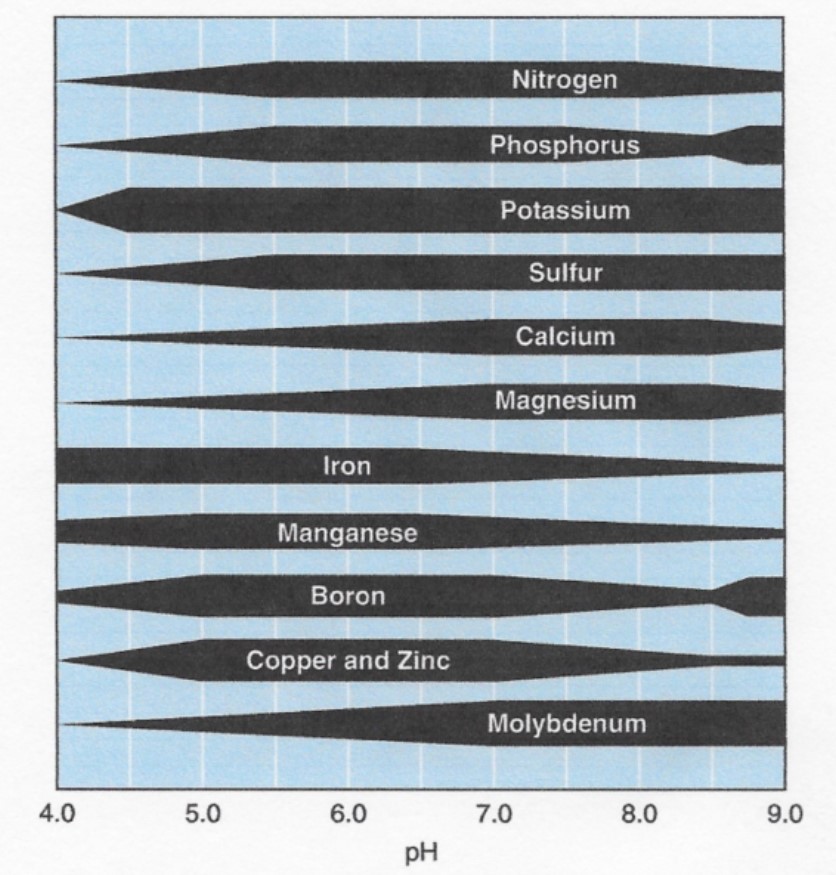
The uptake of different nutrients can vary based on plant growth stages. Research has shown that P, N, Cu, and S have the greatest percentage of total nutrient uptake in the grain during the seed-filling growth stages. Potassium uptake occurs primarily during the late vegetative and early reproductive growth stages.3
Customarily, additional amounts of P and K are applied prior to planting corn to sustain the following year’s soybean crop. The amounts for this application should be based on soil tests, crop removal rates, and realistic yield goals for corn and soybean crops. Healthy soybean plants can produce or fix N from the air into readily available ammonia in their root nodules because of the bacterium Bradyrhizobium japonicum.
The best method for determining current plant-available soil nutrients is to analyze properly collected soil samples. The practical time to collect soil samples is after harvest but prior to the onset of winter-time cold temperatures that can change soil properties physically and chemically. At a minimum, soil samples should be collected every three to four years; annual soil testing provides the best measurement for available nutrients but may not be economical for many operations. Sampling should occur at the same time frame each year.
The number of samples collected should be sufficient to capture variability within each field and be a balance between sampling cost and desired precision. Samples should be taken from the surface to tillage depth (about 6 to 8 inches). In reduced tillage systems, a shallow surface to 1-inch deep sample should be collected along with a surface to 6-inch deep sample because fertility stratification can occur. The samples should be kept and analyzed separately.4
The optimum sampling pattern is based on grid cells of 2.5, 4, or 8 acres. The smaller the cell, the greater the precision. Global positioning system (GPS) technology is used to identify each grid. Within each grid, 5 to 7 cores should be collected, composited, and analyzed individually from the other grids. Based on the results, application rates can be prescribed for each grid and applied with a variable rate applicator. If grid sampling is not practical or economical, 10 to 20 core samples should be collected by using a “Z” or “W” pattern across the defined area, composited, and analyzed separate of other sampling areas. The least representative sampling technique is the composite of one sample from an entire field from cores drawn across the field. To help avoid skewing results for the latter two sampling techniques, samples should not be taken from fertilizer bands, headlands, dead furrows, dusting areas from limestone roads, and areas where stockpiles of P, K, lime, manure, or other elements or composts existed.1,5
Regardless of the collection pattern used, samples should be collected in clean plastic containers as metal containers can contaminate the samples. Probes or augers should be stainless steel and not be coated with Zn, Cu, or other potential contaminants. Properly labeled composited samples should be placed into containers designed for soil samples and sent immediately to a soil lab or allowed to dry in a dust-free environment for later submission. Soil information sheets should be thoroughly completed with information such as cropping history, crop to be planted, tillage methods, soil region, and realistic yield goals based on the past 3 to 5 years. The analysis requested should include macro and micronutrient levels, soil pH, organic matter (OM), soluble salts (salinity), and cation exchange capacity (CEC).
Understanding the Laboratory Results
Laboratory results may be reported in parts per million (ppm) or lb/acre.
To convert ppm to lb/acre, multiply ppm by 2 (lb/acre = ppm X 2).
To convert lb/acre to ppm, divide lb/acre by 2 (ppm = lb/acre ÷ 2).
Nitrogen: Generally, results on N availability are unimportant for soybean production because of the plant’s ability to produce N. Soil available nitrate (NO3-) or ammonium (NH4+) is initially needed for soybean seedling establishment until the nodules begin producing N. If a soybean crop has not been produced for several years, the seed should be inoculated with Bradyrhizobium japonicum bacterium to facilitate nodule growth.
Phosphorus: Plant-available P in the soil solution is related to pH; therefore, labs may use different P extraction methods depending on the soil pH. The Bray P1 (weak Bray) for acidic soils with pH less than 7.0 is used to measure readily available P. The Bray P2 (strong Bray) for highly acidic soils measures readily available P plus a part of the active reserve soil P (useful where rock phosphate has been applied). Mehlich-3 P for acidic soils is used for P, K, Ca, Mg, Na, and micronutrient testing. The Olsen P test is used for neutral to highly alkaline soils (pH of 7.3 or greater) where Ca can tie up P and make it less available for plant growth. Monitoring P levels, applying what is needed, and application methods are important because of potential loss by erosion and/or runoff, which can be problematic for water quality.
Potassium: Three forms of K are in equilibrium in the soil: unavailable, slowly available, and readily available. About 90 to 98% of total soil K contained in feldspars and micas is unavailable. Slowly available K is “fixed” between soil clay layers and may be released when soils become wet; however, soil tests do not provide a value for fixed K. Readily available K is measured by soil tests because of its solubility in water. Submitted soil samples that were air dried may result in an underestimated value for available K because the dried soil “fixes” K. The underestimation can result in a soil report indicating a need for additional K to be applied. For soybean production, broadcasting and incorporating K fertilizer sources prior to planting have proven to be the most effective for deficient soils. Banding can be used in reduced tillage systems.6 Michigan State recommends maintaining soil K levels between 0 and 30 ppm above critical K levels to help maximize soybean yields. The critical level is calculated by multiplying the CEC by 2.5 and adding 75.7
Micronutrients: Most soils have adequate amounts of the micronutrients required for soybean production. However, obtaining soil test values for these nutrients is important so they can be addressed if needed. Deficiencies may be observed more often in coarse textured, muck, and peat soils. Of the micronutrients, iron (Fe) is likely the most important in high pH areas in the Dakotas, Minnesota, Nebraska, and Iowa because iron deficiency chlorosis (IDC) can occur. Soil testing for Fe itself is not generally needed but a determination of soil pH is required to help ascertain if IDC could be problematic.
For additional information on IDC, please see Iron Deficiency Chlorosis
Sulfur may be needed in coarse textured soils that are low in OM because power plant emissions of S have decreased. If S is deficient, it should be applied prior to planting because it is needed early in soybean growth.
Manganese soil deficiencies may be observed in muck or dark-colored sands with pH above 5.8 and lakebed or out wash soils with pH levels above 6.5.7 Broadcasting or banding is not recommended because of high soil fixation and economics (banding). The most economical and effective method to address a soil Mn deficiency is a foliar application of 1 to 2 lb/acre of actual Mn.7
Boron is most likely to be deficient in coarse-textured soils, high OM soils, and lakebed soils having high pH levels. Broadcasting 1 lb/acre (2 lb/acre maximum) of actual B blended with K fertilizer two weeks prior to planting is a method to address a soil deficiency. A foliar application of 0.25 lb/acre (0.5 lb/acre maximum) during early bloom is another method. Exceeding the maximums can potentially cause crop injury through B toxicity.7
Organic Matter: Soil OM affects many soil biological, chemical, and physical properties that can influence nutrient availability.
Soil pH: As defined earlier, the optimal pH range for soybean growth is between 6.3 and 6.5. Soil pH is measured on a logarithmic scale of 0 (acid) to 14 (alkaline). A soil pH of 6 is 10 times more acidic than a soil with a pH of 7 and a pH of 5 is 100 times more acidic than a pH of 7. The availability of different nutrients is greatly dependent on pH levels. Most nutrients are available in a pH range of about 5.5 to 7.0.
Over time, crop production without lime applications can result in a decrease in soil pH (becomes more acid) which decreases the availability of crucial nutrients. Soil testing laboratories use a buffer pH test to determine the amount of lime required to bring the soil back within an acceptable pH range. A large change in buffer pH indicates that soil pH can be changed easily with a low rate of lime whereas a small change indicates that a higher lime rate is needed. The quickness that pH can be changed is dependent on the coarseness and the geographical source of the lime.
Cation Exchange Capacity: This value is a measure of the soil’s capacity to hold positively charged particles such as K+, NH4+, Cu2+, Fe2+, and Mn2+. The capacity to hold is due to the negative charge possessed by clay particles within the soil and OM. A CEC above 10 milliequivalents per 100 grams (10 meq/100 g) is considered adequate.8 Higher CEC values are an indication that the soil contains more clay and/or OM. Higher CEC soils have greater water holding capacities, are able to hold K and Mg more readily, can maintain soil pH longer, and if needed, require more lime to increase soil pH.8 Building the soil’s OM can help increase soil CEC.
Salinity: High soluble salt content (salinity) can cause water stress, nutrient imbalances in plants, and may affect nutrient uptake. Seedlings are sensitive to higher than normal soluble salts. Leaching can cause soil salinity levels to change rapidly; therefore, sampling should occur periodically during the growing season.
For additional information please read Soybean Fertility During the Planning Season
Sources:
1Rogers, E. 2019. The 4R’s of nutrient management. MSU Extension Field Crops. Michigan State University. https://www.canr.msu.edu/.
2Fernandez, F.G. and Hoeft, R.G. 2009. Managing soil pH and crop nutrients. Chapter 8. Illinois Agronomy Handbook. http://extension.cropsci.illinois.edu/.
3Bender, R.R., Haegele, J.W., and Below, F.E. 2015. Modern soybean varieties’ nutrient uptake patterns. Better Crops. Vol. 99. No. 2. http://cropphysiology.cropsci.illinois.edu/.
4Keryk, K., Ketterings, Q., Albrecht, G., Stockin, K., and Beckman, J. Soil sampling for field crops. Agronomy Fact Sheet Series. Fact Sheet 1. Cornell University Cooperative Extension. http://nmsp.cals.cornell.edu/.
5Sawyer, J., Mallarino, A., and Killorn, R. Take a good soil sample to help make good decisions. Iowa State University Extension. PM 287. Revised September 2003.
6Kaiser, D.E. and Rosen, C.J. 2018. Potassium for crop production. University of Minnesota Extension. University of Minnesota. https://extension.umn.edu/.
7Staton, M. 2013. Nutrient management recommendations for high-yield soybean production. Soybean Management and Research Technology (SMART). MSU Extension. Michigan State University. https: www.canr.msu.edu/.
8Ketterings, Q., Reid, S., and Rao, R. 2007. Cation exchange capacity (CEC). Agronomy Fact Sheet Series. Fact Sheet 22. Cornell University Cooperative Extension. http://nmsp.cals.cornell.edu/.
Additional Sources:
Wortmann, C.S., Krienke, B.T., Ferguson, R.B., and Maharjan, B. 2018. Fertilizer recommendations for soybean. NebGuide. G859. Nebraska Extension. University of Nebraska. http://extension.unl.edu/.
Dinkins, C.P. and Jones, C. 2013. Interpretation of soil test results for agriculture. Montana State University Extension. MontGuide. Publication No. MT200702AG.
Web sites verified 10/18/19. 1006_S4
